- Powered by ENDURALIFE™ Battery Technology, which is backed by four independent studies and over six years of real-world data6 that consistently demonstrated industry-leading longevity performance.
- The world’s thinnest ICD designed to enhance patient comfort.
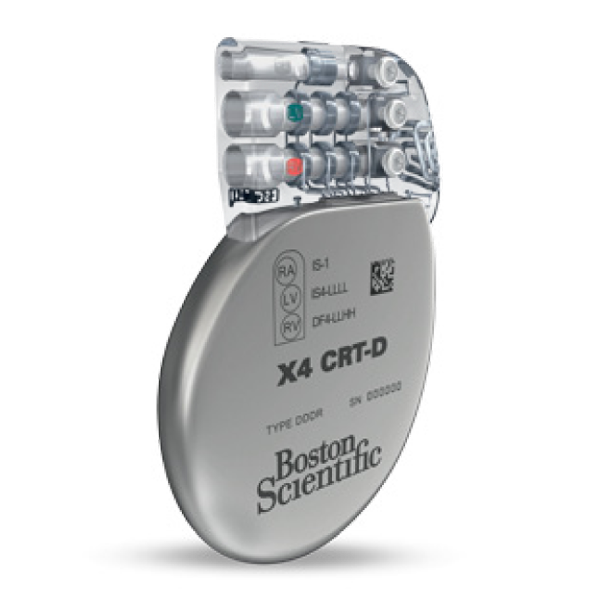
- Includes the new EasyView™ header with color coded lead ports, designed to improve implant efficiency.
- A clear header to easily verify full lead insertion
- First-in-industry color-coded port labels to reduce the potential for misidentification of header ports
ICD INOGEN continuously checks the heartbeat. It delivers electric shocks, when needed, to restore a regular heart rhythm.
An ICD is the main treatment for anyone who has survived cardiac arrest. The devices are increasingly used in people at high risk of sudden cardiac arrest. An ICD lowers the risk of sudden death from cardiac arrest more than medicine alone.
Benefits:
- Projected to last 11+ years for VR devices and 10+ years for DR devices under true usage conditions.
- This small (29.5 cc) and thin (9.9 mm) high-energy device is designed to enhance patient comfort.
- Offers an advanced system solution for patient comorbidities and HF monitoring (HF Perspectiv™ report, LATITUDE™ NXT Remote Patient Management with weight scale and blood pressure sensors, and Respiratory Rate Trend).
- Offers AcuShock™ Technology, multiple programmable options to reduce inappropriate and unnecessary shocks, including a choice of rhythm discriminators, antitachycardia pacing (ATP) therapy in all rates zones, and advanced sensing and filtering.
- AV Search+ and RYTHMIQ™ give clinicians options to appropriately manage RV pacing in patients with varying degrees of conduction block.
- EasyView™ header and color coded lead ports designed to make the implant experience more efficient.
- SafetyCore™ technology is intended to provide lifesaving shock therapy and basic pacing functionality in the event of an unrecoverable fault.
Studies, conclusions and links
- 1. Haarbo J, Hjortshoj S, Johansen J, Jorgensen O, Nielsen J, Petersen H. Device Longevity in Cardiac Resynchronization Therapy Implantable Cardioverter Defibrillators Differs Between Manufacturers: Data from the Danish ICD Registry. Presented at HRS 2014. https://ondemand.hrsonline.org/common/presentation-detail.aspx/15/35/1241/9000. Boston Scientific = 136 patients, Medtronic = 651 patients, St. Jude Medical = 1,587 patients, Bitronik = 369patients. Time to exchange of the device because of battery depletion or device failure recorded in the Danish ICD Registry was the endpoint. The four-year survival rate for devices in the Danish Registry study was 81.1% forMedtronic and 95.7% for Boston Scientific (P<0.01).